Introduction
Primary osteoarthritis knee (OA Knee) is also known as degenerative disease or age-related arthritis. It is found in both the sexes, all over the world and existing since ages. It starts as pain in the knee at about 30 yrs without any evident cause. Pain occurs on joint movement and subsides on rest. Disease progresses over a period of 10-15 yrs when its signs develop at >50 yrs (clinical plus radiographic criteria developed by American College of Rheumatology)1. Despite the age in diagnostic criteria >50, it occurs in adults also- “OA Knee (pain with x-ray evidence) occurs in 12% of persons age ≥60 in United States and 6% of all adults age ≥302. As per the literature, its etiology and pathogenesis are not known- “The exact mechanism for the development of primary OA remains unknown and it is therefore termed idiopathic3. The basic lesion described is degeneration (weakening) of the articular cartilages which progresses with age and aggravates by obesity, diabetes and heredity. OA knee occurs more in those who work in standing or squatting position 4. There are many treatments being used for this disease which are as follows-
A. Non-surgical
1. Physiotherapy, Assistive devices Heat, Electricity5, USG, Laser, Cane, Knee- sleeve
2. Medication NSAIDS, Paracetamol
3. Intra-articular injections1 Hyaluronic Acid, Corticosteroids, Stem cells etc.
B. Surgical
Total knee Arthroplasty (TKA)6,7, Knee Joint Distraction8
There is no cure to this disease9. The treatment is based on pain management and mobility restrictions. All the patients are initially treated with one or more non-surgical options which work for some time. Surgery is advised on failure of this regimen. The most relied operation at present is TKA6,7. This has its own disadvantage as it works for only 15-20 yrs6. So, the question arises what is the specific treatment?
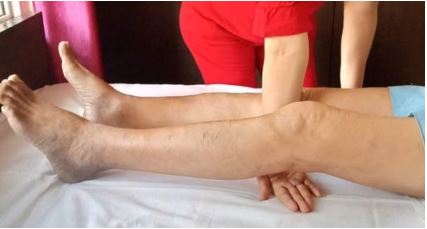
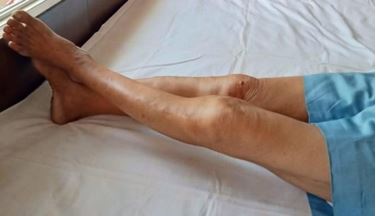
The available literature lacks precise knowledge of its causes, pathogenesis & treatment. This article aims to establish the same and assess the results by a Randomized Controlled Trial (RCT). The hypothesized causes are DFF/DFE/both; pathogenesis is contracture formation in front/back of the capsule and treatment, passive flexion/passive extension knee (CCT). The treatment differs as per the cause & site of contracture.
Material and Methods
Subjects
The total participants included 125 patients (250 joints) of OA Knee. The G1 included 100 and G2, 25. This number depended upon their scanty availability in my setup. Their inclusion criteria consisted of
1. Age 30 to 85 years
2. Knee pain which appeared without any apparent cause, exacerbated by exertion and subsided on rest
3. Limited morning stiffness
4. No past H/O infection, trauma or inflammation (to rule out secondary OA) and
5. Disability in sitting, climbing stairs or walking. The exclusion criteria consisted of
6. Backache
7. Leg pain (e.g. sciatica)
8. Inability to lie supine (e.g. kyphosis)
9. Inability to lie prone (e.g. central obesity)
Exclusion was based on patients’ inability to lie supine and prone, required for the intervention. This trial was based on “Pragmatic Cluster Randomized Controlled Trial”, also known as Cluster Randomized Trial (CRT)13 or Group Randomized Trial. In this variety pre-existing groups, called clusters, of individuals are randomly allocated to treatment arms. CRTs can be used when individual randomization to treatment arm is not possible or the intervention is naturally applied to whole cluster. My patients, who consulted me, were of two types. The type I wanted to avoid surgery, had tried other non-surgical options (e.g. drugs, physiotherapy, and intra-articular injections) and did not want those anymore. In such a situation it was not possible to give them any other intervention except the CCT. So those were included in the trial group. The type II were already using some options and living with disabilities but not convinced to receive CCT. So those were included in control group with “no intervention”.
The proof of control group as valid pre-existing cluster was obtained by following formula:
All primary OA Knee patients = Pre-existing subjects valid for trial
OR
One patient X n (imaginary number) = Pre-existing subjects valid for trial
OR
1 patient X 28 cluster* = Pre-existing subjects valid for trial
OR
All 28 patients cluster = Pre-existing subjects valid for trial hence
My control group (G₂) = Pre-existing subjects valid for trial
The randomization was non-blinding as CCT was unmaskable procedure
The study setting consisted of my clinic, one charitable hospital, free weekly health camps and clinics of two colleagues. The study period was March 2017 - December 2017. This ten month period gave sufficient time of six months for follow up. Informed consent was sought from all patients. The patients with bilateral affection were investigated and treated simultaneously. The procedure consisted of
1. Baseline Data Recording
2. Intervention
3. Data Collection and Monitoring.