Prospective analysis of clinico-radiological efficacy of Trans-foraminal Lumbar Interbody Fusion (TLIF) in degenerative disc disease - Mid term follow up of 2 years
Patidar A1*, Mehta R2, Sharma S3, Singh V4, Chauhan A5
1* Ashish Patidar, Assistant Professor, Department of Orthopaedics, R D Gardi Medical College, Ujjain, Madhya Pradesh, India.
2 R Mehta, Department of Orthopaedics, R D Gardi Medical College, Ujjain, Madhya Pradesh, India.
3 S Sharma, Department of Orthopaedics, R D Gardi Medical College, Ujjain, Madhya Pradesh, India.
4 V Singh, Department of Orthopaedics, R D Gardi Medical College, Ujjain, Madhya Pradesh, India.
5 A Chauhan, Department of Orthopaedics, R D Gardi Medical College, Ujjain, Madhya Pradesh, India.
Background: Low back pain as a result of degenerative disc disease (DDD) imparts a large socioeconomic impact on the health care system. Correct diagnosis and treatment of DDD is difficult and controversial. Whether inter-body fusion is the treatment of choice in DDD is still a dilemma. The Transforaminal interbody fusion (TLIF) developed by Harms is a modification of posterior lumbar interbody fusion(PLIF). Advantage of the TLIF over PLIF with lesser complications avoidance of epidural scarring, less intra-operative bleeding, and lesser chance of dural injury.
Methods: We evaluated 30 patients operated for DDD with TLIF between 2014 to 2017. Patients > 35 years, both sexes, two level or less involvement, degenerative spondylolisthesis (grade I, II), with predominantly low-back pain, with or without radiculopathy or claudication, disability to perform daily activities and not relived by non-operative treatment for at least 6 months were included. All other cases were excluded. Thorough clinical and radiological examination was done.
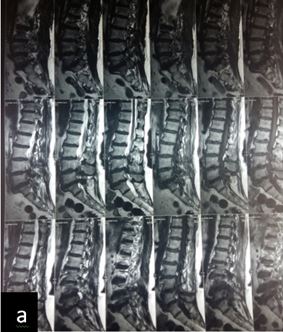
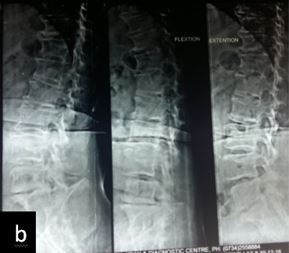
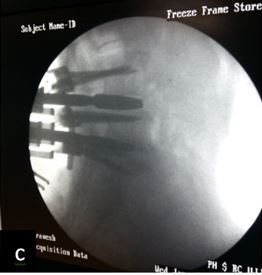
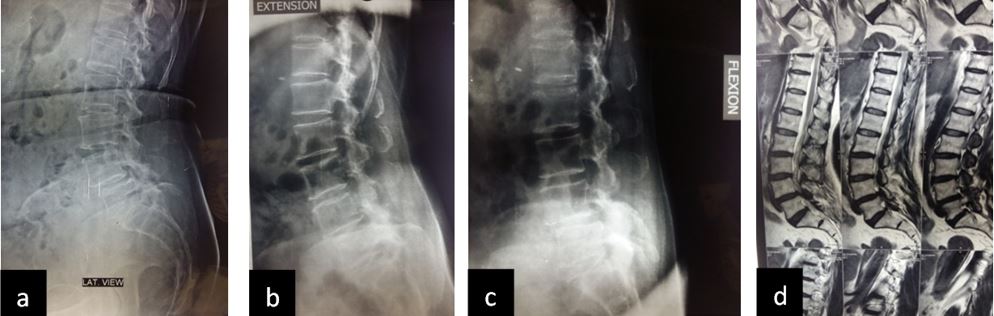
Patient was followed up at 1, 3, 6 and 9 months post-op for X-ray (to see for progress of union), VAS score and ODI index and complications. Bony fusion was assessed by a single radiologist on basis of X-ray only.
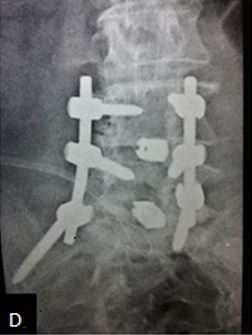
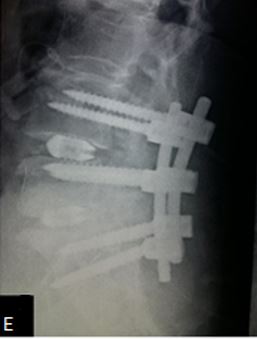
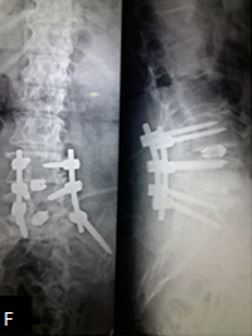
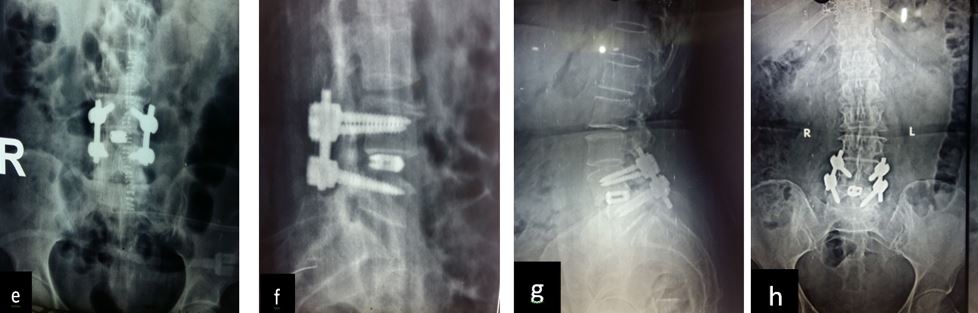
Results: 17 M 13 F patients with 19 patients having instability while 11 not, were evaluated. L4-5 was the most common level. Average pre-operative VAS score was 7.7667 (S.D 1.104) while at last follow-up was 2.133 (S.D 0.434). Average pre-operative ODI was 47.133 (S.D 8.215) while at last follow-up it was 25.533 (S.D 4.191) (Table-2). Mean operative time for one and two level was 97.3 minutes and 143.2 minutes respectively. Average blood loss was 465 ml (390- 580ml). 28 patients had bony fusion at last follow-up (93 %). Two patients who did not show bony fusion were asymptomatic. We encountered intra-operative violation of S1 pedicle in one case, dural puncture in three cases, contra-lateral radiculopathy in one case and asymptomatic adjacent segment disease in 4 cases at final follow-up.
Conclusion: We conclude from our study that TLIF is simpler, easier and safe procedure for Degenerative Disc Disease with good surgical, functional and radiological outcomes.
Keywords: Degenerative disc disease, Interbody Fusion, TLIF
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Assistant Professor, Department of Orthopaedics, R D Gardi Medical College, Ujjain, Madhya Pradesh, India. Email: |
Patidar A, Mehta R, Sharma S, Singh V, Chauhan A, Prospective analysis of clinico-radiological efficacy of Trans-foraminal Lumbar Interbody Fusion (TLIF) in degenerative disc disease - Mid term follow up of 2 years. ojmpc. 2017;23(2):3-10. Available From https://ojmpc.com/index.php/ojmpc/article/view/52 |